


Next: EXPERIMENTAL
Up: X-ray Structure Determination of Fully
Previous: X-ray Structure Determination of Fully
Obtaining the average bilayer structure of the benchmark lipid,
dipalmitoylphosphatidylcholine (DPPC), in the biologically relevant,
fully hydrated, 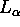 phase has been very challenging.
Estimates for area A per molecule span an unacceptably large range
(Nagle, 1993) from 58Å
phase has been very challenging.
Estimates for area A per molecule span an unacceptably large range
(Nagle, 1993) from 58Å to 71Å
to 71Å and there are corresponding
uncertainties in bilayer thickness. These uncertainties have inhibited
understanding of the biophysical differences between bilayers composed of
different lipids. Reducing this uncertainty and obtaining additional
structural data for DPPC also will provide more stringent tests of the potentials
employed in molecular dynamics simulations of membranes.
and there are corresponding
uncertainties in bilayer thickness. These uncertainties have inhibited
understanding of the biophysical differences between bilayers composed of
different lipids. Reducing this uncertainty and obtaining additional
structural data for DPPC also will provide more stringent tests of the potentials
employed in molecular dynamics simulations of membranes.
One of the major problems in determining the structure of DPPC
bilayers in the 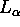 (synonymously the fluid, chain melted or liquid-crystalline)
lamellar phase is the small number of observable orders of diffraction when the sample
is fully hydrated. More orders of diffraction can be observed at
reduced hydration levels, but the most direct explanation for this is
that the bilayer structure changes with hydration.
If this explanation were correct, then the strategy of
reducing the hydration level to obtain bilayer structure would
seem to be biologically equivocal, since biological membranes are
usually fully hydrated.
(synonymously the fluid, chain melted or liquid-crystalline)
lamellar phase is the small number of observable orders of diffraction when the sample
is fully hydrated. More orders of diffraction can be observed at
reduced hydration levels, but the most direct explanation for this is
that the bilayer structure changes with hydration.
If this explanation were correct, then the strategy of
reducing the hydration level to obtain bilayer structure would
seem to be biologically equivocal, since biological membranes are
usually fully hydrated.
On the other hand, the loss of higher orders of diffraction with increasing hydration
could be due to increased undulation fluctuations, which also systematically
reduce the size of the higher order peaks that one can measure.
The theory for this has been carefully developed (Zhang et al., 1994)
and we have obtained synchrotron data at high instrumental resolution that verify
that the peak shapes are well described by the theory
(Zhang et al., 1995a and 1995b). One of the main results of this paper is
the presentation of the corrected form factors for many different
D spacings. These form factors fit well on a single continuous transform
F(q) as is required if the bilayers do not change structure upon
dehydration. This suggests that some very
careful previous studies on partially dehydrated samples
(e.g. Buldt et al, 1979; Wiener and White, 1992)
may be appropriate for the biologically relevant fully hydrated 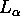 phase.
phase.
A standard way to analyze low angle x-ray structural data is to plot the
Fourier reconstruction from the form factors for the observable peaks
(McIntosh and Simon, 1986a and 1986b). A primary result from such plots is the
head-head spacing 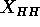 . Our theory (Zhang et al., 1994)
shows that
. Our theory (Zhang et al., 1994)
shows that 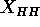 changes very little when corrections due to
fluctuations are made and this will be illustrated with the
present data. The largest change due to fluctuation corrections is to
sharpen the features in the electron density profiles.
changes very little when corrections due to
fluctuations are made and this will be illustrated with the
present data. The largest change due to fluctuation corrections is to
sharpen the features in the electron density profiles.
The preceding improvements culminate in electron density profiles that
should be useful in comparing to molecular dynamics simulations and that
give a measure of the head-head spacing 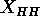 . Although the latter is often
interpreted as the phosphate-phosphate distance, this may not be literally
true, and it is certainly not possible to obtain hydrocarbon thicknesses
nor interfacial areas A from electron density profiles, even in the
gel phase (Wiener et al, 1989). In the case of the gel phase, one has
additional information from the sharp wide angle peaks, such as
chain area
. Although the latter is often
interpreted as the phosphate-phosphate distance, this may not be literally
true, and it is certainly not possible to obtain hydrocarbon thicknesses
nor interfacial areas A from electron density profiles, even in the
gel phase (Wiener et al, 1989). In the case of the gel phase, one has
additional information from the sharp wide angle peaks, such as
chain area 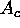 and tilt angle
and tilt angle 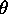 (Tristram-Nagle et al., 1993;
Sun et al., 1994) that enable a fairly complete average structure
determination. For DLPE bilayers McIntosh and Simon (1986b) showed how to
obtain the structure of the
(Tristram-Nagle et al., 1993;
Sun et al., 1994) that enable a fairly complete average structure
determination. For DLPE bilayers McIntosh and Simon (1986b) showed how to
obtain the structure of the 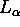 phase, especially
the evasive area of the fluid phase
phase, especially
the evasive area of the fluid phase 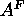 , by using gel phase structure
together with measured differences between the gel and fluid phases
in headgroup spacing
, by using gel phase structure
together with measured differences between the gel and fluid phases
in headgroup spacing 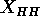 and lipid volumes.
Essentially the same procedure is applied in this paper
for the structure of the
and lipid volumes.
Essentially the same procedure is applied in this paper
for the structure of the 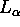 phase of DPPC.
phase of DPPC.



Next: EXPERIMENTAL
Up: X-ray Structure Determination of Fully
Previous: X-ray Structure Determination of Fully
This document was converted to HTML by Nikolai Gouliaev on Fri Oct 4 15:28:39 EDT 1996
Your comments are welcome at gouliaev@andrew.cmu.edu
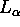 phase has been very challenging.
Estimates for area A per molecule span an unacceptably large range
(Nagle, 1993) from 58Å
phase has been very challenging.
Estimates for area A per molecule span an unacceptably large range
(Nagle, 1993) from 58Å to 71Å
to 71Å and there are corresponding
uncertainties in bilayer thickness. These uncertainties have inhibited
understanding of the biophysical differences between bilayers composed of
different lipids. Reducing this uncertainty and obtaining additional
structural data for DPPC also will provide more stringent tests of the potentials
employed in molecular dynamics simulations of membranes.
and there are corresponding
uncertainties in bilayer thickness. These uncertainties have inhibited
understanding of the biophysical differences between bilayers composed of
different lipids. Reducing this uncertainty and obtaining additional
structural data for DPPC also will provide more stringent tests of the potentials
employed in molecular dynamics simulations of membranes.
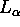 (synonymously the fluid, chain melted or liquid-crystalline)
lamellar phase is the small number of observable orders of diffraction when the sample
is fully hydrated. More orders of diffraction can be observed at
reduced hydration levels, but the most direct explanation for this is
that the bilayer structure changes with hydration.
If this explanation were correct, then the strategy of
reducing the hydration level to obtain bilayer structure would
seem to be biologically equivocal, since biological membranes are
usually fully hydrated.
(synonymously the fluid, chain melted or liquid-crystalline)
lamellar phase is the small number of observable orders of diffraction when the sample
is fully hydrated. More orders of diffraction can be observed at
reduced hydration levels, but the most direct explanation for this is
that the bilayer structure changes with hydration.
If this explanation were correct, then the strategy of
reducing the hydration level to obtain bilayer structure would
seem to be biologically equivocal, since biological membranes are
usually fully hydrated.
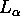 phase.
phase.
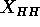 . Our theory (Zhang et al., 1994)
shows that
. Our theory (Zhang et al., 1994)
shows that 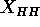 changes very little when corrections due to
fluctuations are made and this will be illustrated with the
present data. The largest change due to fluctuation corrections is to
sharpen the features in the electron density profiles.
changes very little when corrections due to
fluctuations are made and this will be illustrated with the
present data. The largest change due to fluctuation corrections is to
sharpen the features in the electron density profiles.
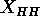 . Although the latter is often
interpreted as the phosphate-phosphate distance, this may not be literally
true, and it is certainly not possible to obtain hydrocarbon thicknesses
nor interfacial areas A from electron density profiles, even in the
gel phase (Wiener et al, 1989). In the case of the gel phase, one has
additional information from the sharp wide angle peaks, such as
chain area
. Although the latter is often
interpreted as the phosphate-phosphate distance, this may not be literally
true, and it is certainly not possible to obtain hydrocarbon thicknesses
nor interfacial areas A from electron density profiles, even in the
gel phase (Wiener et al, 1989). In the case of the gel phase, one has
additional information from the sharp wide angle peaks, such as
chain area 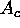 and tilt angle
and tilt angle 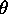 (Tristram-Nagle et al., 1993;
Sun et al., 1994) that enable a fairly complete average structure
determination. For DLPE bilayers McIntosh and Simon (1986b) showed how to
obtain the structure of the
(Tristram-Nagle et al., 1993;
Sun et al., 1994) that enable a fairly complete average structure
determination. For DLPE bilayers McIntosh and Simon (1986b) showed how to
obtain the structure of the 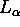 phase, especially
the evasive area of the fluid phase
phase, especially
the evasive area of the fluid phase 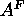 , by using gel phase structure
together with measured differences between the gel and fluid phases
in headgroup spacing
, by using gel phase structure
together with measured differences between the gel and fluid phases
in headgroup spacing 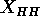 and lipid volumes.
Essentially the same procedure is applied in this paper
for the structure of the
and lipid volumes.
Essentially the same procedure is applied in this paper
for the structure of the 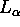 phase of DPPC.
phase of DPPC.